Here is an interesting press release on the use of liposomes to treat lung infections. This may be the key to controling bleeding in racehorses, who probably are infected with pulmonary biofilms:
Monday, March 31, 2008 STUDY CONFIRMS ANTI-INFECTIVE ARIKACE™ EFFECTIVELY PENETRATES MUCUS AND BIOFILM, AND KILLS PSEUDOMONAS, A BACTERIA PLAGUING CYSTIC FIBROSIS PATIENTS
MONMOUTH JUNCTION, NJ, March 31, 2008 Transave Inc. reported today that its lead product candidate, ARIKACE™ (liposomal amikacin for inhalation), may have the ability to penetrate mucus and biofilms, and decrease the number of Pseudomonas aeruginosa lung infections in patients with cystic fibrosis, according to results of a study published in the Journal of Antimicrobial Chemotherapy.
Results of the study titled "Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomanas aeruginosa lung infections," were published in the April issue of the journal. Transave is a biopharmaceutical company focused on the development of next-generation liposomal drug products for inhalation.
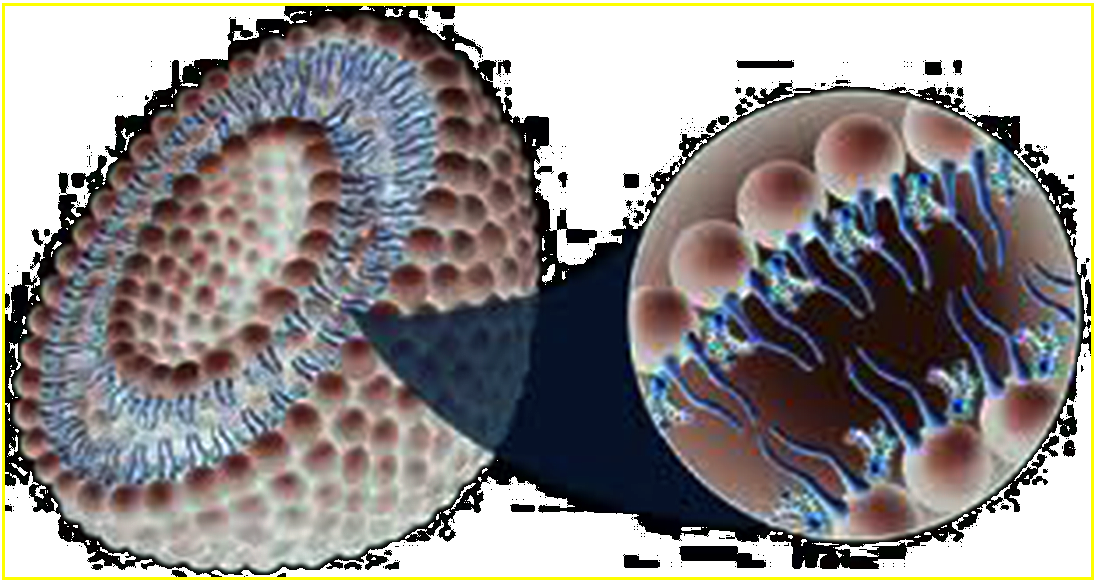
ARIKACE is a form of the antibiotic amikacin, which is enclosed in nanocapsules of lipids called liposomes. The study evaluated the ability of the ARIKACE liposomes to pass through patient mucus (sputum) ex vivo and to penetrate the bacteria's biofilm barrier in an established flow-cell in vitro model. The biofilm is a gel-like matrix in the lungs formed by colonies of Pseudomonas that create a protective barrier for bacteria. This prevents patients with cystic fibrosis (CF) from clearing infections even under aggressive antibiotic treatment. It is not practical to observe biofilm interactions in humans; therefore, the Center for Biofilm Engineering at Montana State University has developed a model to microscopically visualize the penetration of liposomes into Pseudomonas biofilms.
Separate studies showed that the small, neutrally charged ARIKACE liposomes facilitated antibiotic passage through the patient mucus layer and penetration into the Pseudomonas biofilm. Prior studies have shown that free aminoglycosides bind to patient mucus and thus have reduced bioactivity in killing Pseudomonas. Using microscopic techniques, the investigators were able to see that Arikace liposomes effectively penetrated and lodged into the spaces within biofilms, where the antibiotic can be released very close to the bacteria.
Another important study enabled investigators to identify bacterial virulence factors secreted by Pseudomonas in the biofilm that trigger release of amikacin from the liposomes. These factors are expected to be concentrated in and near the colonies of Pseudomonas growing in the biofilm within the static mucus of CF lungs. This area is an ideal target for antibiotics and the triggered release of amikacin resulted in what was essentially Pseudomonas suicide.
"Pseudomonas is insidious in the lungs of CF patients who live with chronic infection which is why we developed a liposomal delivery technology small enough for nebulization and able to penetrate the highly-shielded biofilm," said Walter Perkins, Chief Technology Officer at Transave. "Our ultimate goal is to deliver more active antibiotic locally to the site where Pseudomonas resides in the lung, and sustain it there, while reducing the treatment burden with fewer daily doses of drug therapy."
"We know for sure that the liposomes were interacting with, and accumulating in, the biofilm," said Dr. Garth James, Ph.D., director of medical projects at the Center for Biofilm Engineering at Montana State University and a co-author of the study. Montana State University's Center for Biofilm Engineering is the world's largest and oldest biofilm research center.
"You need a drug that can penetrate biofilm in order to kill the infection. This unique drug delivery mechanism may allow that to happen," Dr. James said. "By using an advanced delivery mechanism, you can keep the drug where it needs to be for a longer period of time. A "free" drug inhaled into the lungs would likely be cleared more rapidly."
Excess mucus in the lungs of CF patients is a breeding ground for insidious Pseudomonas bacteria. CF patients with these types of infections are typically treated twice daily for 28 days with an inhaled antibiotic followed by a 28 day drug holiday or "off period." This on/off cycle is often repeated multiple times and patients are surviving longer as a result. However, the commercially available treatments are not designed to penetrate the patient mucus and biofilm, have limited exposure time, and are not able to kill some of the Pseudomonas bacteria protected by the biofilm. The remaining bacteria quickly reproduce during the off treatment period to pretreatment levels and may be more resistant to further antibiotic treatment. This cycle leaves CF patients in a chronic state of infection and can cause further development of hyper-mutated or mucoidal strains of Pseudomonas. Respiratory exacerbations, loss of lung function and death can result.
The study was authored by Drs. Paul Meers and Walter Perkins of Transave Inc., and their colleagues, in collaboration with Garth James, Ph.D., and Steven Fisher, Ph.D., of the Center for Biofilm Engineering at Montana State University. The publication is currently available on the journal's website (jac.oxfordjournals.org/cgi/content/abstract/61/4/859).
The Cystic Fibrosis Foundation provided a $1.7 million award for the clinical program. The Cystic Fibrosis Foundation is the leading organization devoted to curing and controlling cystic fibrosis.
About ARIKACE (liposomal amikacin for inhalation)
ARIKACE is a form of the antibiotic amikacin that is enclosed in nanocapsules of lipid called liposomes. This proprietary next-generation liposomal technology prolongs release of amikacin in the lung while minimizing systemic exposure. The treatment uses biocompatible lipids endogenous to the lung that are formulated into small (0.3 mm) neutrally charged liposomes that enable biofilm penetration and are highly efficient with very low lipid to drug ratio (0.6). ARIKACE can be effectively delivered through nebulization where the small aerosol droplet size (~3.0 mm) facilitates lung distribution. Two Phase II studies are currently being conducted in patients that have CF and Pseudomonas lung infections in Europe and the United States. An abstract on the top line European Phase II data in CF has been accepted for presentation at the 31st European Cystic Fibrosis Conference in Prague in June. ARIKACE has been granted orphan drug status in the United States by the FDA and orphan drug designation in Europe by the European Medicines Agency (EMEA) for the treatment of Pseudomonas infections in patients with CF.
About Transave Inc.
Transave Inc is a biopharmaceutical company focused on the development of innovative, inhaled pharmaceuticals for the site-specific treatment of serious lung diseases. The company's major focus is developing antibiotic therapy delivered via next-generation liposomal technology in areas of high unmet need in respiratory disease. Transave is dedicated to leveraging its advanced liposomal development and commercialization expertise, along with its intellectual property, to bring life extending and enhancing medicines to patients.